 |
|
|
 |
| |
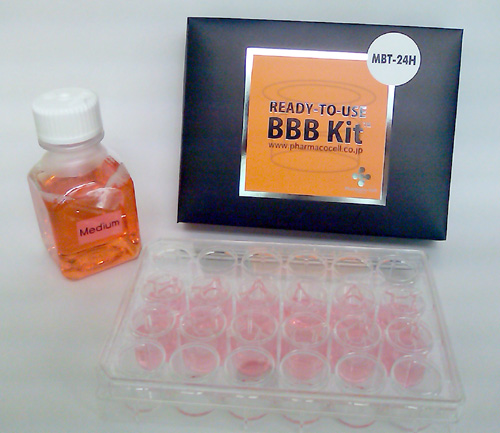
| Newly Released Monkey BBB Kit™ |
| MBT-24H (F*) |
 |
 |
| *On-Demand Product |
| RBT-24H (F*) |
 |
 |
| *On-Demand Product |
| RBE-12 (PET-12) |
 |
|
 |
|
|
BBB
(Zurich) 2010
[PDF-2.15MB] |
|
 |
 |
|
BBB
(Potsdam) 2007
[PDF-652KB] |
CVB 2007
[PDF-1.2MB] |
|
 |
|
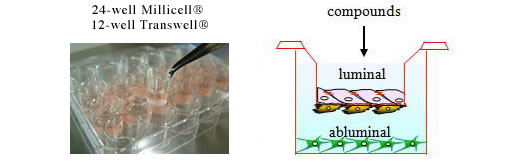
The new, ready-to-use BBB Kit™ is a significant product of PharmaCo-Cell Company Ltd.; an in vitro blood-brain barrier (BBB) model for drug transport assays, and research on BBB pathophysiology (Nakagawa et al., 2007, 2009). This unique model uses three cell types, primary cultures of monkey (Macaca irus) or rat (Wistar rat) brain capillary endothelial cells, brain pericytes and astrocytes. This BBB Kit™, reconstituted by triple co-culture, shows many of the in vivo BBB features. The model can be used for drug BBB permeability assay , as well as basic BBB researches (Fig.1).
|
|
|
- comes from monkey (MBT-24) or rat (RBT-24 & RBE-12) BBB-related cells
- comes in frozen, can be stored at -80℃
- comes in 24-well Millicell® (MBT-24 & RBT-24) and 12-well Transwell® (RBE-12) format
- can be used shortly after receiving
- does not require cell passaging or long culture
- is flexible, filter membrane can be selected depending on the research purpose
- is saving time and money, no need for the difficult and expensive primary culture techniques
|
|
| Main applications of ready-to-use BBB Kit ™ |
- Drug BBB permeability assay
- Research on BBB physiology: cell-cell interactions, transport pathway modulations
- Research on BBB toxicology: brain endothelial toxicity assays
- Research on BBB pathology: disease modeling
|
|
|
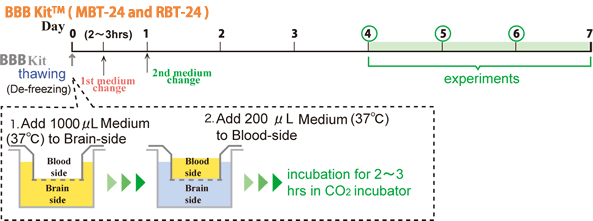
We deliver the complete set of BBB Kit in frozen packaged with dry ice. BBB Kit can be frozen as a whole and stored at -80℃.
4 days prior to your experiment, you just thaw (de-freeze) your BBB Kit stored at -80℃.
 |
| |
| |
Amounts of solutions (to be added)
| |
Thawing-sol |
medium (1) |
medium (2) * |
| Blood-side |
200 μL |
300 μL |
300 μL |
| Brain-side |
1000 μL |
1200 μL |
1200 μL |
* Recipe: medium 10% PDS / DMEM F-12
| |
Dulbecco's Modified Eagle's Medium/ Nutrient Mixture F-12 Ham (DMEM F-12) |
|
| Fetal Bovine Plasma Derived Serum (PDS) |
10% (v/v) |
| Heparin |
100 μg/mL |
| basic fibroblast growth factor (bFGF) |
1.5 ng/mL |
| Insulin-Transferrin-sodium Selenite (ITS) |
insulin 5 μg/mL,
transferrin 5 μg/mL,
sodium selenite 5 ng/mL |
| Hydrocortisone |
500 nM |
| Gentamicin |
50 μg/mL |
|
|
|
| Description of ready-to-use BBB Kit ™ |
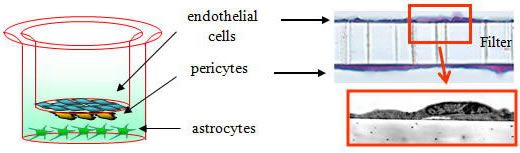
The ready-to-use BBB Kit™, an in vitro reconstituted BBB model is composed of three cell types (Fig. 2). Primary rat brain pericytes are seeded on the lower side of the tnserts (Fig. 2A). Primary rat astrocaytes cultures are plated at the bottom of the 12-or 24-well culture dishes (Fig. 2B). Monolayers of primary monkey or rat brain capillary endothelial cells are cultured on the upper side of the same Millicell® ( MBT-24 / RBT-24 ) or Transwell® inserts (RBE-12) (Fig. 2C) (Nakagawa et al., 2007, 2009). This innovative and new model is very close to the actual anatomical situation, since the BBB is formed in vivo by the dynamic interaction of these cell types (Nakagawa et al., 2009).
A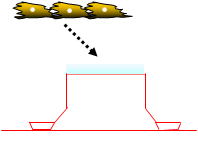 |
B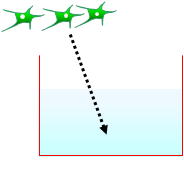 |
C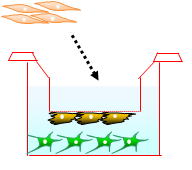 |
D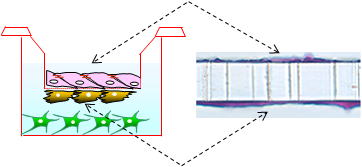 |
| Fig. 2. Brain pericytes are plated at the lower side of inserts (A), astrocytes at the bottom of the wells (B), and brain endothelial cells at the upper side of the same inserts (C) to reconstitute the BBB in vitro: Schematic drawing of the Kit, and crossection of the insert with endothelial layer (top) and pericytes (bottom) stained with hematoxyline-eosin (D). |
|
|
| Culture and characterization of the cells of ready-to-use BBB Kit ™ |
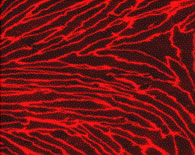
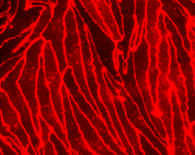
Primary cultures of monkey and rat brain capillary endothelial cells are prepared from 3-year-old monkeys (Macaca irus) and 2-3 week-old Wistar rats according to the protocol described (Nakagawa et al., 2007, 2009), respectively. In the first two days, culture medium contains puromycin (4 μg/ml) to selectively remove P-glycoprotein negative contaminating cells (Perriere et al., 2005, 2007). Confluent monolayers of primary brain capillary endothelial cells in BBB Kit™ give positive immunostainings for von Willebrand factor (vWF) or factor VIII endothelial marker, tight junction protein occludin, ZO-1 and claudin-5.
Monkey BBB Kit™ (MBT-24)
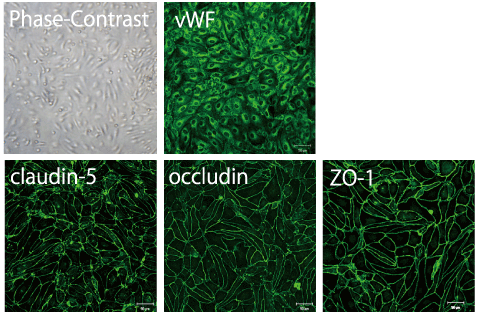 |
| Fig. 3. Expression of von Willebrand factor (vWF), ZO-1, occludin and claudin-5 in primary monkey brain capillary cells |
|
Rat BBB Kit™ (RBE-12)
 |
 |
 |
| Factor VIII |
ZO-1 |
claudin-5 |
| Fig. 4. Expression of Factor VIII, ZO-1 and claudin-5 in primary rat brain capillary cells |
|
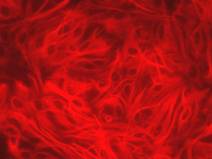
Primary cultures of glial cells are prepared from the newborn Wistar rats (Nakagawa et al., 2007). In confluent glia cultures 90 % of cells are immunopositive for the astrocytes marker glial fibrillary acidic protein (GFAP), while the remaining 10 % is immunopositive for CD11b, a marker of microglia. Primary rat brain pericytes are cultured according to the method of Nakagawa et al.(2007, 2009) and contain characteristic α-smooth muscle actin.
|
 |
 |
| GFAP |
α-smooth muscle actin |
| Fig.4. Expression of GFAP in primary rat astrocytes and α-smooth muscle actin in primary rat pericytes |
|
|
|
| Characterization of ready-to-use BBB Kit ™ |
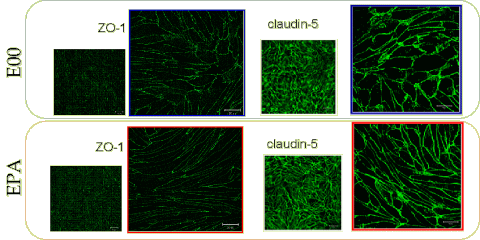
Astrocytes and brain pericytes help to develop and maintain specific BBB characteristics in brain capillary endothelial cells (Abbott et al., 2006; Deli et al., 2005). Co-culture of the three cell types in BBB Kit™ led to the enhancement of barrier properties; an increase in expressions of tight junction proteins of occludin, claudin-5 and ZO-1 and continuous localizations of ZO-1 and claudin-5 (Fig. 6).
| A. |
 |
| B. |
 |
| Fig. 6. Expression of tight junction proteins in rat brain endothelial cells in mono-culture (E00) and in BBB Kit™ (RBE-12) (EPA) on a immunohistochemistry (A) and a Western blot (B): Localization of claudin-5 and ZO-1 of tight junction proteins in rat brain endothelial cells are shown. |
|
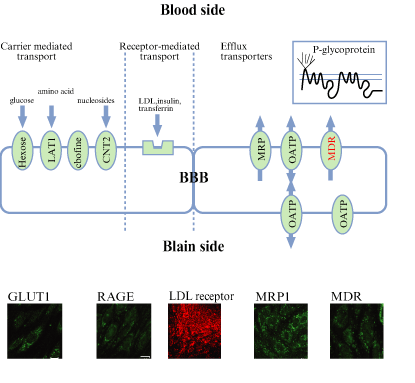
| Fig. 7. Expressions of BBB transporters in BBB Kit™ (RBE-12) |
|
|
|
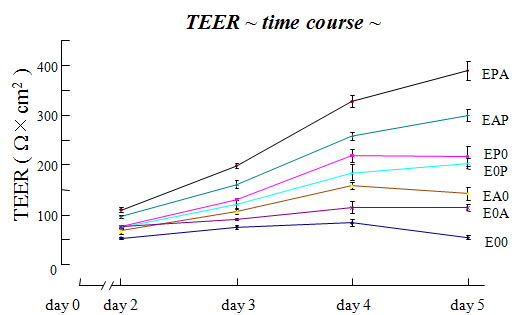
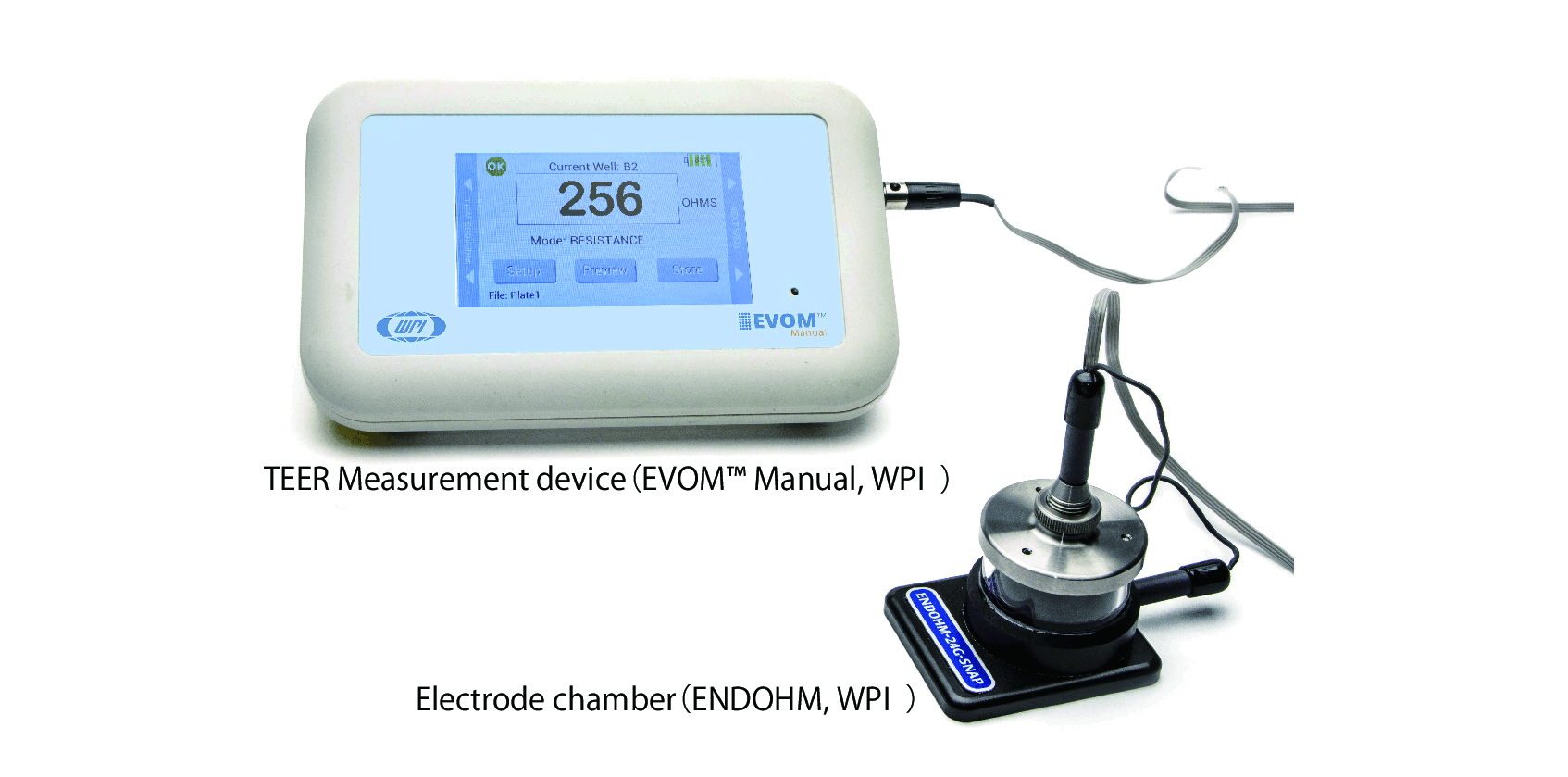
| Trans Endothelial Electrical Resistance (TEER) data |
| Tight Junction Function |
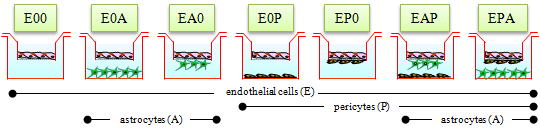
7 types of BBB models (Nakagawa et al., 2007) |
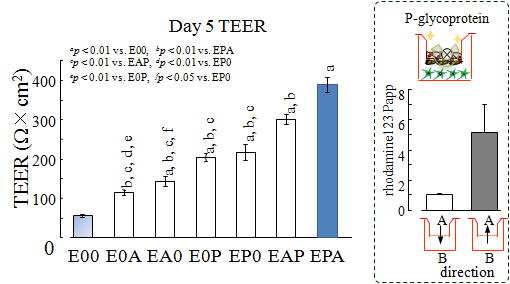 |
| Fig. 8. TEER in BBB Kit™ (RBE-12) |
|
|
|
【Equipment】
 |
| Permeability data |
| Paracellular transport / sodium fluoresceine (Na-F, 376Da) |
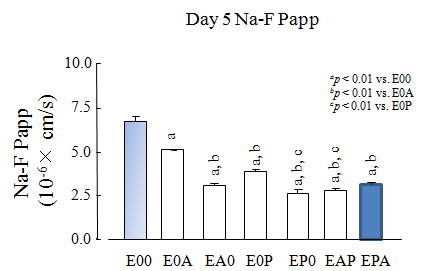 |
| Fig. 9. Papp of sodium fluoresceine (Na-F) (RBE-12) |
|
|
| BBB Permeability assay using BBB Kit ™ |
When testing the penetration of a molecule (permeability marker, receptor ligand, drug candidate etc.) through the brain endothelial and pericytes layer of BBB Kit™ representing the BBB, in a blood-to-brain direction, the molecule is applied to the upper (luminal, blood-side) compartment of the insert (Fig. 10). Transport is measured after a given time (Fig. 9, ⊿T), either a single time point (e.g. 5 min) or multiple time points (e.g. 20, 40, 60 min) by detecting the amount of compound from the lower (basal, brain-side) compartment. BBB Kit can also be used for detecting the efflux transport in an inverse direction.
*See details in Drug Permeability Assay
|
|
| Fig. 10. Transport of a molecule (blue) through BBB Kit™ in a luminal to basal direction |
|
|
| Calculation of apparent permeability (Papp in cm/min) |
Apparent permeability coefficient Papp (in cm/min) based on the Fick's law can be calculated according to the following equation;
| 1. |
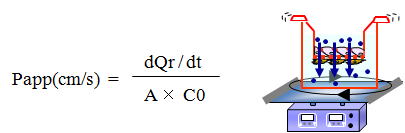 |
| 2. |
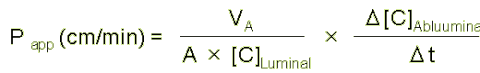 |
|
VA: volume of abluminal chamber (cm3)
A: membrane surface area (1.12 cm2)
[C]L: initial luminal tracer concentration (ng/ml)
[C]A : abluminal tracer concentration (ng/ml)
⊿t : time of experiment (min) |
|
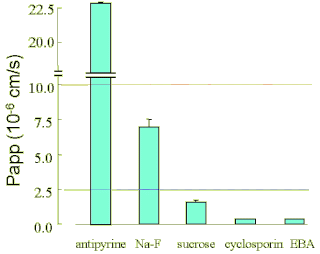 |
| Fig.11. Papp caluculated from the data obtained with BBB Kit™ (RBE-12) |
|
| BBB Kit™ (MBT-24/RBT-24) and (RBE-12) |
BBB Kit™ (MBT-24) and (RBT-24) are with a polyethylene terephthalate ( PT ) membrane filter (3.0 µm pores), and are especially good for examinations of drug-permeability through the BBB. BBB Kit™ (MBT-24) and (RBT-24) can be also available for BBB permeability of macromolecules such as proteins and peptides, and of cells. Because of polycarbonate membrane filter, endothelial cells grown on the filter cannot be observed with inverted microscope, a phenomenon which occasionally causes a difficulty to perform researches for BBB-physiology and -pathology.
BBB Kit™ ( RBE-12 ) is equipped with a polyester ( PE ) membrane filter (0.4 µm pores), and is good for researches for BBB-physiology and -pathology, and for examinations of drug-permeability through the BBB. Because of chemical characteristics of polyester, some substances absorbs to the membrane, a phenomenon which occasionally causes a difficulty in calculating the substance's BBB permeability. |
| Properties of Millicell® insert (MBT-24/RBT-24) and Transwell® insert (RBE-12) in BBB Kit™ |
Inserts & Membranes (PDF-74KB) (PDF-74KB) |
|
BBB Kit™ (MBT-24/RBT-24) |
BBB Kit™ (RBE-12) |
| Product company |
Millipore Corporation |
Corning Incorporated |
| Product name |
Millicell® Hanging Cell Culture Insert |
Transwell® Permeable Support |
| Catalog Number |
#PISP 12R 48
 |
#3460
 |
| Membrane material |
Polyethylene Terephthalate |
Polyester |
| Pore Size (μm) |
3.0 |
0.4 |
| Membrane Diameter (mm) |
6.5 |
12 |
| Membrane Surface Area (cm2) |
0.33 |
1.12 |
| Apical Volume (μL) |
200/300 |
500 |
| Basolateral Volume (μL) |
900/1200 |
1500 |
| Height of insert (mm) |
16 |
- |
| Pore Density (pores/cm2) |
2 x 106 |
4 x 106 |
| Membrane Thickness (μm) |
9 |
10 |
| Optical Property |
Translucent |
Clear |
| Cell visibility |
Poor |
good |
|
|
| Applications of ready-to-use BBB Kit ™ |
- Transport and permeability studies from ions to macromolecules: effect of physiological or pathogenetic factors
- Paracellular barrier and cell polarity studies: TJ protein expression, distribution, polarized distribution of transport proteins, receptors, enzymes etc
- Studies on endo- and transcytosis, receptor-ligand interactions
- Drug transport, drug effect on permeability, localization of receptors, polarity of drug responses
- Co-culture studies: cell-cell and cell-matrix interactions
- Microbial pathogenesis: virus, bacteria, parasite attachment, invasion and penetration
|
|
|
Millicell® is the Registered Trade Mark of Millipore Corporation.
Transwell® is the Registered Trade Mark of Corning Incorporated. |
REFERENCES:
Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T,
Kittel A,
Tanaka K, Niwa M: A new blood-brain barrier model using primary rat brain
endothelial cells, pericytes and astrocytes. Neurochemistry International 54: 253-263, 2009. doi:10.1016/j.neuint.2008.12.002
Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa
M: Pericytes from brain microvessels strengthen the barrier integrity in primary
cultures of rat brain endothelial cells. Cellular and Molecular Neurobiology 27(6):678-694, 2007 Open Access Open Access
Deli MA: Blood-brain barrier models. In: Lajtha, A.(Ed.), Handbook of Neurochemistry and Molecular Neurobiology, Neural Membranes and transport. vol. 11, Springer, New York, pp.29-56, 2007
Deli MA, Ábrahám CS, Kataoka Y, Niwa M: Permeability studies on in vitro blood-brain barrier models: Physiology, Pathology, and Pharmacology. Cellular and Molecular Neurobiology 25:59-127, 2005
Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, Niwa M: Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regulatory Peptides 123:77-83, 2004
Dohgu S, Takata F, Yamauchi , Nakagawa S, Egawa T, Naito M, Tsuruno T, Sawada Y, Niwa M, Kataoka Y: Brain pericyes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-β production. Brain Research 1038:208-215, 2005
Perrie`re N, Demeuse PH, Garcia E, Regina A., Debray M., Andreux, J-P, Couvreur P, Scherrmann J-M, Temsamani J, Couraud P-O, Deli MA, Roux F: Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. Journal of Neurochemistry 93:279-289, 2005
Perrie`re N, Yousif S, Cazaubon S, Chaverot N, Bourasset, F, Cisternino S, Decle`ves X, Hori S, Terasaki T, Deli M, Scherrmann J-M, Temsamani J, Roux F, Couraud P-O: A functional in vitro model of rat blood-brain barrier for molecular analysis of efflux transporters. Brain Research 1150:1-13, 2007
Abbott NJ, Ro¨nnba¨ck L, Hannson E: Astrocyte-endothelial interactions at the blood−brain barrier. Nature Reviews Neuroscience 7:41-53, 2006
|
|
|
|
 |
 |
|
|
